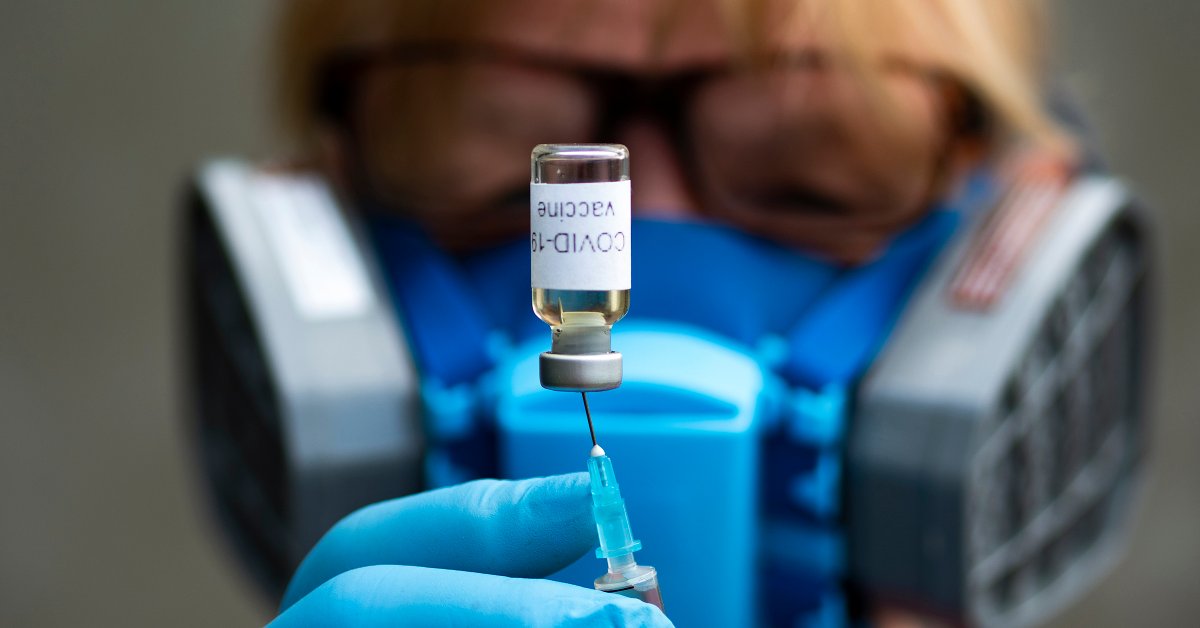
Vaccines by themselves do not save lives, but rather the immunisation process does. This highlights a challenge the world is facing right now. The process used to create a vaccine in a laboratory is different to the one used for bulk manufacturing by the pharmaceutical industry in order to immunise people.
We already manufacture billions of doses of vaccines every year, from the annual influenza jab to the combined measles, mumps and rubella (MMR) immunisation. The 2009 swine flu pandemic, which saw the death of hundreds of thousands of people, lead to approximately three billion doses of flu vaccines being produced and delivered within six months.
Developing a new vaccine is a lengthy process. The 2014 to 2016 Ebola outbreak in West Africa resulted in the death of more than 11,000 people. Scientists with the Public Health Agency of Canada had been working on the rVSV-ZEBOV vaccine since 2003 and it was only during the Ebola outbreak that the vaccine’s clinical trials were actually conducted. It was successfully concluded in November 2016 and was approved three years later following a further trial study with 15,000 people.
Creating and manufacturing an entirely new vaccine on a national or global scale, whilst maintaining production of all the other vaccines, becomes a Herculean endeavour, experts say. “We’re making a vaccine for a virus that we’ve never made a vaccine for, that’s not approved, using platforms that haven’t been used extensively in the clinic with patients,” explains Angela Rasmussen, a virologist at Columbia University’s Center for Infection and Immunity.
Conventionally, vaccine research can take 10 years from initial development through to mass distribution. However, for Covid-19, there is a global effort to reduce that to only 18 months, whilst maintaining the same safety standards.
Central to this is the parallel development process that the pharmaceutical industry is using during the crisis. Vaccine research is usually performed in a sequence of successive steps. Typically, there would be a laboratory development stage, followed by animal testing, and then several stages of clinical trials. Once these stages have been successfully completed, the vaccine would be submitted for approval, prior to developing a manufacturing process.
For Covid-19 vaccines, several stages of development are being carried out in parallel. This is necessary due to the urgent need for the vaccine, but it has the drawback that one stage of the process does not inform the next in the usual way. For example, results from animal trials would normally inform the choice of appropriate dose level for the start of human trials. The present situation is that data from several various stages are being analysed concurrently.
“We’re actually seeing preclinical data in non-human primates for vaccines that are already in phase three clinical trials,” explains Margaret Liu, chair of the board of the International Society for Vaccines.